Scientific Qi Exploration – Part 13(a)
Qigong and the Immune System
— The Innate Immune System
Marty Eisen, Ph.D.
 1. Introduction to the Immune System
1. Introduction to the Immune System
To understand the effects of Qigong on the immune system a brief review will be presented (1). The review will also indicate that there are many aspects of the immune system whose relationship to Qigong still remains to be investigated.
The immune system of the body resists invasion by foreign organisms, eliminates these organisms, neutralizes and eliminates potentially damaging agents produced by these organisms. It also surveys the body for harmful internal elements, such as modified or cancerous cells, and tries to eliminate such perceived threats. Thus, the immune system usually has beneficial effects. However, in some situations, the immune response can be harmful. It can attack bodily tissues resulting in autoimmune diseases, such as, Crohn’s disease and Type I Diabetes. Its interaction with pathogens can lead to inflammation resulting in the release of toxins causing collateral damage to healthy tissues. Infection by an organism does not necessarily lead to a disease. Disease occurs when there are an inordinate number of virulent pathogens or immunity is weakened.
Immunity depends on the ability to distinguish between self and non-self. Every cell in the body carries the same set of distinctive surface proteins, called the major histocompatibility complex (MHC) proteins, which distinguish it as self. There are two classes: MHC Class I proteins, which are on all cells with a nucleus, and MHC Class II proteins, which are only on certain specialized cells. Any non-self substance, capable of triggering an immune response, is known as an antigen. An antigen can be a whole non-self cell, a bacterium, a virus, an MHC marker protein or even a portion of a protein from a foreign organism. The distinctive markers on antigens that trigger an immune response are called epitopes.
Disease resistance results from two types of defenses, working together, called innate immunity or non-specific resistance and acquired immunity or specific resistance. Each of these two major immune subdivisions has both a cellular and humoral component, whose defense mechanisms depend on cells and biochemical compounds, respectively. In addition, the innate immune system uses anatomical and physiological barriers to block infection.
The adaptive immune system responds by reacting to an antigen via the humoral or cellular divisions and frequently both. There is a time lag between exposure and the maximal response. In a humoral response, specific plasma proteins are synthesized, called antibodies, which are capable of combining with the provoking antigen. The cellular response results in the activation of specific sensitized cells (lymphocytes) to defend against the provoking antigen. Hence, this immunity is also called specific immunity. Exposure results in immunologic memory, the capacity of the body’s immune system to remember an encounter with an antigen due to the activation of specialized cells having specificity for the antigen and to react more swiftly to the antigen by means of these activated cells in a later encounter.
Innate immunity operates in the same way for most invaders (2). It blocks the entry of pathogens into the body or destroys the invaders through many non-specific factors. There is an immediate maximal response, which does not depend on antigens. There is no immunologic memory as a result of exposure to a particular organism.
2. Anatomical, Mechanical, Physiological and Chemical Resistance to Infection
a) Anatomic Barriers
Skin
The skin is a protective cover of the body that prevents the entry of some pathogens. It is usually dry, acidic, and at a lower temperature than the body (98.7F). These conditions are unfavorable to the growth of some bacteria. The continual sloughing of dead, surface cells gets rid of adhering infectious organisms. The first skin layer of epithelial cells produces defensins, small peptides toxic to bacteria and cathelicidins, proteins which are cleaved into two peptides toxic to microbes. Hair follicles and sweat glands produce lysozyme, an enzyme that breaks down bacterial walls and toxic lipids that can kill bacteria. Hair follicles also secrete sebum containing lactic and fatty acids both of which inhibit the growth of some harmful fungi and bacteria. Hairless areas of the skin like the palms and soles are more prone to fungal infection, like athlete’s foot.
Bones
Bony encasements, like the skull and rib cage, protect vital organs from injury and entry of pathogens.
Mucus Membranes
Mucus is a sticky mixture of mucin, a glycoprotein, and water. It is secreted by mucus glands in membranes lining body cavities that open to the exterior, like the respiratory, the gastrointestinal (gi.), and the genitourinary tracts. It can trap the pathogen or parasite and so keep them from reaching the tissues. Mucus also contains the following:
(i) lysozyme (also called, muramidase) can damage bacterial cell walls.
(ii) an antibody (called secretory IgA) that prevents microbes from attaching to mucosal cells, trapping hem in the mucus,
(iii) lactoferrin is a protein which binds iron, keeping it from being used by microbes. It has antibacterial, antiviral, antifungal, anti-inflammatory, antioxidant and immunomodulatory activities;
(iv) lactoperoxidase, an enzyme to generate toxic superoxide radicals that kill microbes.
The mucous membrane is constantly sloughing cells which remove attached microbes.
b) Mechanical Mechanisms
Coughing and sneezing removes mucus and trapped microbes. Vomiting and diarrhea expel pathogens and toxins in the gastrointestinal tract. Peristalsis and defecation also remove invaders. Fluids such as urine, tears, saliva, perspiration, and blood from injured blood vessels also flush bacteria and toxins from the body. Cilia in the respiratory tract propel mucus and microbes upwards towards the throat where they are swallowed. The microbes are killed in the stomach.
c) Biological Mechanisms
Normal flora are microbes that live in every niche of the body with usually no harmful effects. By competing with pathogens for nutrients or attachment to epithelial cells, normal skin organisms can prevent colonization of infectious organisms. They produce substances (bacteriocidins, defensins, cationic proteins, and lactoferrin) which destroy other space competing bacteria. Lactic acid is produced from glycogen by some vaginal bacteria. This creates an unfriendly acidic environment for some foreign invaders.
Semen contains spermine and zinc which can destroy some pathogens. Mother’s milk contains lactoperoxidase, an enzyme that functions as a natural antibiotic.
d) Chemical Mechanisms
Sweat glands secretes lactic and pyruvic acids to produce an acidic environment that retards the growth of some bacteria. Sweat also contains fatty acids, which can also inhibit bacterial growth. The stomach’s acidity will kill most ingested pathogens. The lungs and g.i. tract contain defensins, low molecular weight proteins, that are natural antibiotics. The lungs also contain surfactant (see following Note) which acts as an opsosin, an antibody that causes bacteria or other foreign cells to become more susceptible to the action of phagocytes, cells that ingests microorganisms or other cells and foreign particles. Saliva, tears, and nasal secretions contain the enzymes phospholipase and lysozyme, which can breakdown bacteria’s cell walls.
Note on Surfactant
Surfactant is produced in the lungs from six lipids (fats) and four proteins. Surfactant reduces the surface tension of fluid in the lungs and helps make the small air sacs in the lungs (alveoli) more stable. This keeps them from collapsing when an individual exhales. Fetuses begin making surfactant while still in the womb, in preparation for breathing air. Babies that are born very prematurely often lack adequate surfactant and must receive surfactant replacement therapy immediately after birth in order to breathe.
e) Internal Defenses
Some internal defenses are: temperature, normal flora, acidity, oxygen, and the coagulation system.
The normal temperature of the body inhibits some pathogen’s growth. Fever can also be beneficial to retard the growth of others. Enzymatic reactions, including those of the phagocytes, go faster at the slightly elevated temperatures. Also the increased temperature increases the bacterial requirement for iron, but at the same time makes less of it available. However, very high fevers are detrimental.
Normal flora are another defense.
The stomach secretes hydrochloric acid and protein-digesting enzymes that destroy many pathogens. The bacteria in the intestines also face an acidic environment from various secretions such as, bile acids.
The presence of oxygen can retard the growth of some bacteria and kill others. On the other hand, lack of oxygen is harmful to aerobic organisms, which can survive and grow in an oxygenated environment.
Tissue damage and infection lead to leakage of vascular fluid, containing antibacterial serum proteins and attracts phagocytes. Tissue thromboplastin and specific chemicals secreted by these phagocytes cause the conversion of fibrinogen, the soluble final factor in the clotting cascade, to fibrin, forming clots. Invading microbes can be trapped in blood clots. Some products of the coagulation system have the ability to increase vascular permeability and attract phagocytes. Also, some products of the coagulation system are natural antibiotics. For example, beta-lysine, a protein produced by platelets during coagulation, acts as a cationic detergent. The positively charged components of the cationic detergent molecule attract the negatively charged lipid membrane of the bacterial cell and destroy it. After the invaders have been killed an anti-clotting mechanism is activated to dissolve clots.
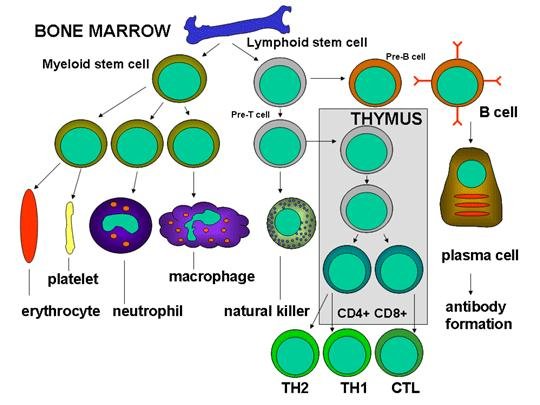
3. Cells of the Immune System (See Fig. 1)
Immune system cells originate in the bone marrow from myeloid or lymphoid stem cells. The myeloid stem cell gives rise to erythrocytes, platelets, neutrophils, basophils, eosinophils, macrophages, monocytes and dendritic cells. The lymphoid stem cell gives rise to the natural killer (NK) cells, T cells and B cells. Further T cell development requires the precursor T cells to migrate to the thymus. There they undergo differentiation into two distinct types of T cells, the CD4+ T helper cell and the CD8+ pre-cytotoxic T cell. Two types of T helper cells are produced in the thymus the TH1 cells, which help the CD8+ pre-cytotoxic cells to differentiate into cytotoxic T cells, and TH2 cells, which help B cells, differentiate into plasma cells, which secrete antibodies.
Monocytes originate from precursors called monoblasts. They circulate in the bloodstream for about one to three days and then move into tissues throughout the body. There they differentiate into tissue resident macrophages or dendritic cells. Macrophages (big eater from Greek) are aptly named because they are phagocytes. The name dendritic cell is derived from the fact that as they grow branched projections or dendrites. They act as messengers between the innate and adaptive immune systems by processing antigen material and presenting it on their surface to certain innate immune cells.
Mast cells are another type of immune cell resembling basophils. Both are thought to originate from bone marrow precursors. The basophil is mature upon leaving the bone marrow, but the mast cell circulates in an immature form and only matures upon reaching a tissue site. This tissue site probably determines its precise characteristics.
More properties and functions of immune cells will be presented in the discussions of cellular innate immunity, in Section 5 and cellular acquired immunity in Part 13 (b).
Immune cells circulate in the blood. . Blood is composed of 38–48% cells and 52–62% liquid plasma. The plasma is 91.5% water. It also transports 7% protein (consisting of albumins (54%), globulins (38%), fibrinogen (7%), and other proteins (1%)) and 1.5% other solids. Blood is slightly alkaline and a little heavier than water.
Hematopoiesis is the process by which all blood cells are manufactured from stem cells in the bone marrow (3). The stem cells produce hemocytoblasts that differentiate into the precursors for the three different types of blood cells: erythrocytes (red blood cells, RBCs), leukocytes (white blood cells, WBCs), and thrombocytes (platelets).
There are two types of leukocytes: granulocytes (containing large granules in the cytoplasm) and agranulocytes (without granules). The granulocytes consist of neutrophils , eosinophils, and basophils (See Table 1). They are also called polymorphonuclear leukocytes (PMN or PML) because of the varying shapes of the nucleus, which is usually lobed into three segments. The agranulocytes are lymphocytes (consisting of B cells and T cells) and monocytes. Lymphocytes circulate in the blood and lymph systems, and make their home in the lymphoid organs. Some of the blood, but not red blood cells (RBCs), pass through the capillaries into the interstitial fluid.
WBCs live 5-9 days and RBCs live for about 120 days. About 2,400,000 RBCs are produced each second! Eventually RBCs migrate to the spleen and die. The spleen scavenges usable proteins from their remains. Healthy females have a little fewer RBCs than a healthy male.
The ABO grouping of RBCs is characterized by the presence or absence of A and/or B antigens on the surface of the RBCs. Blood type AB means both antigens are present whiloe type O means both antigens are absent. Type A blood has only A antigens and type B blood has only B antigens.
| Red Blood Cells | 5.0*106/mm3 | ||
| Platelets | 2.5*105/mm3 | ||
| Leukocytes | 7.3*103/mm3 | ||
| Neutrophil | 50-70% | ||
| Lymphocyte | 20-40% | ||
| Monocyte | 1-6% | ||
| Eosinophil | 1-3% | ||
| Basophil | 0.5–1.0% | ||
Table 1 Healthy Adult Blood Count
4. Innate Humoral Immunity
Some innate humoral defenses have already been discussed in Section 2. Others will be presented in this section.
a) Complement System
This system is the major innate humoral defense system. Its name was derived because its actions complemented the actions of antibodies in defending the body. It consists of about 25 plasma proteins that are synthesized in the liver; these are either enzymes or binding proteins, but circulate in the bloodstream in an inactive form. The classical pathway is a sequence of chemical reactions involving these proteins which is only activated by the binding of antibody molecules to a foreign particle. More details will be given in Part 13 (b) on acquired immunity. The important pathway for innate humora immunity is the alternate pathway. It is activated directly by the invader and is antibody-independent.
Once complement is activated it can produce increased vascular permeability, recruitment of phagocytic cells; lysis and opsonization of bacteria.
b) Interferon
Interferon is any member of a family of similar proteins. When a virus invades a cell, it produces interferon because of the presence of viral nucleic acids. Then, the interferon is secreted into the extracellular fluid. The circulating interferon binds with receptors on other cells. These cells now synthesize viral blocking enzymes that can break down viral messenger RNA (mRNA). The enzymes remain inactive until the cell is invaded by the virus whose nucleic acid activates the enzymes. Interferon does not prevent invasion, but only prevents the virus from taking over the machinery of the host cell. Also, interferon can enhance macrophages’ phagocytic activity, enhance the activity of natural killer (NK) and cytotoxic T cells, and slow cell division which , suppresses tumor growth.
c) Iron
Some bacteria require high concentrations of iron. Lactoferrin and transferrin, a blood plasma glycoprotein, bind iron. This reduces the available supply to these bacteria and so prevents them from reproducing. Plasma iron concentration is also reduced by altering iron metabolism (see e) in the liver, spleen, and elsewhere.
d) Histamine
Phagocytic secretions promote the release of histamine from mast cells, causing vasodilatation and increased capillary permeability.
e) Kinins
Kininogens, inactive precursor proteins in the plasma that have been synthesized by the liver, are split by phagocytic secretions into kinins. These kinins can reinforce the vascular changes induced by histamine, act as strong chemotaxins, stimulate steps in the complement system, and activate pain receptors.
f) Kallikrein
Kallikreins are a group of proteolytic enzymes present in various glands, lymph, urine, and blood plasma. They can also be produced directly from mast cells, neutrophils and basophils, or indirectly from activated platelets. Its major action is the liberation of kinins from kininogen. It also aids in the clotting sequence to generate the enzyme plasmin from the protein plasminogen. Plasmin degrades many blood plasma proteins, most notably, fibrin clots.
g) LEM/EP/LAF ( 4 )
Endogenous pyrogen (EP), also known as leukocyte endogenous mediator (LEM), has recently been demonstrated to be equivalent to lymphocyte-activating factor (LAF). LEM/EP/LAF not only stimulates lymphocyte proliferation in response to antigen but also induces fever and elicits neutrophilia and granulopoiesis and evokes profound cellular, organ, and systemic alterations in trace metals, nitrogen, and hormone distribution and metabolism. These findings suggest that LEM/EP/LAF modulates interactions between nonspecific and specific immunity. This leads not only to the development of immunity but also function to limit tissue injury at the sites of microorganism-phagocyte interaction and facilitate wound healing place metabolic demands on the host which lead to increased excretion of some nutrients, the development of negative nutrient balances and, in the absence of adequate nutrient input, eventual malnutrition.
h) Tumor necrosis factor (TNF, cachexin or cachectin and formally known as tumor necrosis factor-alpha,TNF-A)
TNF-A is a cytokine, a family of proteins, peptides or glycoproteins that carry signals between cells, and thus have an effect (i.e. historically related to growth, and cytokinesis) on other cells. Most organs of the body appear to be affected by TNF-A. It possesses both growth stimulating properties and growth inhibitory processes, and appears to have self regulatory properties. For example, TNF-A induces neutrophil proliferation during inflammation, but it also induces neutrophil apoptosis upon binding to the TNF-R55 receptor. It is produced by several types of cells, but especially by macrophage.
Low levels of the TNF-A may aid in maintaining homeostasis by regulating the body’s circadian rhythm and promote the remodeling or replacement of injured and senescent tissue by stimulating fibroblast growth. TNF-A also has a role in the immune response to bacterial, and certain fungal, viral, and parasitic invasions and in the necrosis of specific tumors. It serves as a key intermediary in the local inflammatory immune response. TNF-A initiates a cascade of cytokines and increases vascular permeability; hence, recruiting macrophage and neutrophils to a site of the infection. TNF-A secreted by the macrophage causes blood clotting which serves to contain the infection.
i) Acute-phase Proteins
Acute-phase proteins are any protein whose plasma concentration increases (or decreases) by 25% or more during certain inflammatory disorders. Some acute-phase proteins are: C-reactive protein (CRP), serum amyloid A (SAA), fibrinogen, and alpha 1-acid glycoprotein. The level of CRP in blood plasma can rise as high as 1000-fold with inflammation. Conditions that commonly lead to marked changes in CRP include infection, trauma, surgery, burns, inflammatory conditions, and advanced cancer. Moderate changes occur after strenuous exercise, heatstroke, and childbirth. Small changes in CRP occur after psychological stress and in several psychiatric illnesses. Marked rises in CRP reflect the presence and intensity of inflammation, but is not an indicator of a particular disease..
i) Interleukin 1 (IL-1)
IL-1 is a protein with numerous immune system functions, including activation of resting T cells, endothelial cells, and macrophages; mediation of inflammation; and stimulation of the synthesis of lymphokines, collagen, and collagenases. IL-1 can also induce fever, sleep, adrenocorticotropic hormone release, and nonspecific resistance to infection. A number of interleukin proteins with varying immune-response properties exist and are identified by numbers of 1 through 8.
j) Fibronectin
Fibronectin is a glycoprotein. One isoform circulates in plasma and acts as an opsonin; another is a cell-surface protein that mediates cellular adhesive interactions.
k) Adrenocortical cortisol .
Adrenocortical cortisol may exert some anti-inflammatory activity at physiological levels. It probably serves to modulate stress-activated immune responses and keep them within tolerable limits.
5. Innate Cellular Immunity
Chemical distress signals, produced at the infection site, attract circulating PMNs and monocytes. These signals are generated from sources such as: N-formyl-methionine containing peptides released by bacteria, clotting system peptides, complement products and cytokines released from tissue macrophages that have encountered bacteria in tissue. Some of these signals stimulate endothelial cells near the infection site to express cell adhesion proteins such as ICAM-1(Inter-Cellular Adhesion Molecule 1) and selectins which bind to components on the surface of phagocytic cells, causing them to adhere to the endothelium. Vasodilators produced at the site of infection loosen the junctions between endothelial cells. This allows phagocytes to cross the endothelial barrier by diapedesis, “squeezing” between the endothelial cells. Once in the tissue spaces some of the distress signals attract phagocytes to the infection site by chemotaxis, movement toward an increasing chemical gradient. These signals also activate phagocytes, which results in increased phagocytosis and intracellular killing of the invading organisms.
Cells of the innate immune system recognize broad molecular patterns found in pathogens but not in the host. They recognize broad patterns of bacterial lipopolysacchrides, peptidoglycans, bacterial DNA, doble-stranded RNA (dsRNA), and others. They lack a high degree of specificity of the adaptive immune system. The broad molecular patterns recognized by the innate immune system are called pathogen associated molecular patterns (PAMPs). The receptors for PAMPS on the cell membranes are called pattern recognition receptors (PRRs). A PRR binds to the PAMP on the surface of the invader like a key fitting into a lock. A particular PRR can recognize a molecular pattern that may be present on a number of different pathogens enabling the receptor to recognize a variety of different pathogens.
The following cells are the main line of defense of the innate cellular immune system.
i) Polymorphonuclear cells (PMNs), at the infection site phagocytose invading organisms, killing them intracellularly. PMNs also contribute to collateral tissue damage that occurs during inflammation.
ii) Tissue macrophages and monocytes, which differentiate into macrophages, also function in phagocytosis and intracellular killing of microorganisms. Macrophages can also kill infected or altered self target cells extracellularly. In addition, macrophages rid the body of necrotic debris and contribute to tissue repair.
After digesting a pathogen, a macrophage will integrate the pathogen’s antigen into the cell membrane and display it attached to an MHC class II molecule. This indicates to other white blood cells that the macrophage is not a pathogen, despite having antigens on its surface. Macrophages, with the aid of helper T cells, play a crucial role in presenting antigen to the adaptive immune system. Eventually, the antigen presentation results in the production of antibodies, which attach to the antigens of pathogens. Some pathogens are very resistant to adhesion by macrophages. The attached antibodies make it easier for a macrophage to adhere to the pathogen by means of the corresponding attached antigen on its cell membrane and so phagocytize the pathogen.
.iii) Eosinophils can damage large extracellular parasites like schistosomes. Activated eosinophils release their granule components including eosinophil peroxidase (a cationic hemoprotein), and eosinophil cationic protein (a ribonuclease which is an eosinophil-specific toxin that is very potent at killing many parasites).
iv) Natural killer (NK) cells are capable of killing virus-infected and malignant target cells but not too efficiently. NK cells become lymphokine-activated killer (LAK) cells upon exposure to IL-2 and IFN-gamma, a member of the interferon family. LAK cells can kill malignant cells efficiently. Continued exposure to IL-2 and IFN-gamma enables the LAK cells to kill transformed as well as malignant cells. LAK cell therapy is one approach for the treatment of malignancies.
NK and LAK cells have two kinds of receptors on their surface: a killer activating receptor (KAR) and a killer inhibiting receptor (KIR). When the KAR encounters its ligand, a killer activating ligand (KAL) on the target cell the NK or LAK cells are capable of killing the target. However, if the KIR also binds to its ligand then killing is inhibited even if KAR binds to KAL. The ligands for KIR are MHC-class I molecules. Thus, if a target cell expresses class I MHC molecules it will not be killed by NK or LAK cells even if the target also has a KAL which could bind to KAR. Normal cells constitutively express MHC class I molecules on their surface, however, virus infected and malignant cells down regulate expression of class I MHC. Thus, NK and LAK cells selectively kill virus-infected and malignant cells while sparing normal cells.
v) Killer (K) cells are not a morphologically distinct type of cell. Rather a K cell is any cell that mediates antibody-dependent cellular cytotoxicity (ADCC). In ADCC antibody acts as a link to bring the K cell and the target cell together to allow killing to occur. K cells have an Fc receptor on their surface for the Fc part of the antibody. Thus, they can recognize, bind and kill target cells coated with antibody. Killer cells which have Fc receptors include NK, LAK, and macrophages which have an Fc receptor for IgG type antibodies and eosinophils which have an Fc receptor for IgE type antibodies.
Phagocytic cells have a variety of receptors on their cell membranes through which infectious agents bind to the cells, such as: Fc, complement, scavenger, and toll-like receptors. Scavenger receptors recognize and uptake large molecule having a negative charge as well as modified Low-density lipoprotein (LDL), one of the 5 major groups of lipoproteins that enable lipids like cholesterol to be transported within the blood. TLRs are a type of PRR and recognize molecules that are broadly shared by pathogens but distinguishable from host molecules (PAMPs). After attachment of a bacterium to a PRR, the phagocyte begins to extend pseudopods around the bacterium. The pseudopods eventually surround the bacterium and engulf it. The bacterium is enclosed in a phagosome, a membrane-bound vacuole. The cytoplasm of phagocytes contains lysosomes, membrane-bound vesicles filled with digestive enzymes. The lysosomes fuse with the phagosome and empty their contents in it and so destroy the bacterium.
During phagocytosis there is an increase in glucose and oxygen consumption which is called the respiratory burst. In the respiratory burst a number of oxygen-containing compounds are produced which kill the phagocytized bacteria. This is referred to as oxygen-dependent intracellular killing.
PMNs and macrophages have means to protect themselves from the toxic oxygen intermediates. These reactions involve the dismutation of superoxide anion to hydrogen peroxide by superoxide dismutase and the conversion of hydrogen peroxide to water by catalase.
Bacteria can also be killed by pre-formed substances released from lysosomes when they fuse with the phagosome, called oxygen-independent intracellular killing. Some of these substances are: cationic proteins (cathepsin), which can damage bacterial membranes; lysozyme, which breaks down bacterial cell walls; lactoferrin, which chelates iron depriving bacteria of this required nutrient; hydrolytic enzymes, which break down bacterial proteins. Thus, even patients who have defects in the oxygen-dependent killing pathways are able to kill bacteria. However, since the oxygen-dependent mechanisms kill more efficiently, patients with defects in these pathways are more susceptible to infections and can take longer to recover.
The binding of bacteria to macrophages, especially by Toll-like receptors, results in the production of TNF-A. This induces the expression of the nitric oxide synthetase gene (i-nos) resulting in the production of nitric oxide (NO). If the cell is also exposed to interferon gamma (IFN-gamma) additional nitric oxide will be produced. Nitric oxide released by the cell is toxic and can kill microorganism in the vicinity of the macrophage.
References
1. Male, D. et al. Immunology. Ed. 7, Elsevier Health Sciences, 2006
2. http://pathmicro.med.sc.edu/ghaffar/innate.htm
3. http://uhaweb.hartford.edu/BUGL/immune.htm#blood
4. Powanda, M.C. and Beisel, R.W. Hypothesis: leukocyte endogenous mediator! endogenous pyrogen/lymphocyte-activating factor modulates the development of nonspecific and specific immunity and affects nutritional, Am. J. of Clinical Nutrition, 35, pp 762-68, 1982.

Pingback: Scientific Qi Exploration-13(c)- Qigong and Immunity | Yang-Sheng